Vero Cell Vaccine Efficacy - Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn
Purified Vero cell rabies vaccine is safe carries a very low adverse reaction rate and is effective in preventing rabies in severely exposed subjects when used with human or equine rabies. Analysis was by intention to treat.

Sinovac Reports Positive Data On Covid 19 Booster Loss Of Antibodies Within Months 2021 08 10 Bioworld
The World Health Organization WHO has refuted the claims that a mix and match regimen of Vero Cell and AstraZeneca CoviShield vaccines would yield.

Vero cell vaccine efficacy. COVID-19 Vaccine Vero cell Inactivated. The panel found clarifications provided with regard to regulatory and administrative information to be satisfactory. Vaccine efficacy for symptomatic and hospitalized disease was estimated to be 79 all age groups combined.
Serbia looks east to fill coronavirus vaccine shortage So far Vero is the only Chinese vaccine for which the manufacturer has published official data. This vaccine candidate reported protection in two live viral non-human primate and hamster challenge models. The panel submitted the review report titled SARS-CoV2 vaccine vero cell inactivated manufactured by Beijing Institute of Biological Products to Dr.
Kamal Jayasinghe NMRAs Chief Executive Officer on March 17 2021. Vaccine Efficacy 10000 CI 95 2033-10000 Efficacy against symptomatic COVID-19 cases in Indonesia Efficacy after 14 days with two doses of vaccination Data as of January 11 2021. B Chicken embryonic fibroblasts CEFs and Vero cells were infected with a multiplicity of infection MOI of 10 and collected 24 h postinfection hpi.
Vaccine efficacy against hospitalization was 79. Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell to Prevent COVID-19 in Healthy Adult Population In Peru Healthy Adult Population In Peru Covid-Peru The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. On the basis of all available evidence WHO recommends the vaccine for adults 18 years and older in a two-dose schedule with a spacing of three to four weeks.
The efficacy rating followed an interim report of ongoing human trials conducted in that country CNBC reported. Generation of vaccine. Cell nuclei were counterstained with DAPI blue.
Of studiesstarting rating 7 RCTs1 4 Factors decreasing confidence Limitation in. What is the effectiveness of two doses of inactivated Vero cell-derived JE vaccine in preventing JE disease in individuals living in JE-endemic areas. Referring to the above principles the SARS-CoV-2 inactivated vaccine Vero cells was administered once each on days 0 14 28 and 42 for a total of four administrations and the interval was 2 weeks followed by a recovery period after the last administration of 2 weeks 14 days.
Bharat Biotech has developed a vero cell-based whole-virion inactivated severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 vaccine BBV152 formulated with alum and a TLR78 agonist producing a T-helper-1 cell skewed response. On December 29 2020 Sinopharm reported 79. A Study to Evaluate The Efficacy Safety and Immunogenicity of Inactivated SARS-CoV-2 Vaccines Vero Cell in Healthy Population Aged 18 Years Old and Above COVID-19 The safety and scientific validity of this study is the responsibility of the study sponsor and investigators.
The study is registered with ClinicalTrialsgov number NCT00566345. Rating Adjustment to rating t No. The primary objective was the efficacy of the vaccine in preventing cell-culture-confirmed influenza infection with viruses that were antigenically matched to one of the vaccine strains.
The primary study objective was the efficacy of Vero-cell-derived influenza vaccine in prevention of culture-confirmed infection with influenza virus that was antigenically matched to one of the vaccine. PNGase F was used for. A study by Public Health England PHE found in May the Pfizer PFEN -BioNTech 22UAyDE vaccine was 88 effective against symptomatic disease.
The COVID-19 vaccine under development by Chinas Sinopharm is showing efficacy of 86 health authorities from the United Arab Emirates reported this morning. A large multi-country Phase 3 trial has shown that 2 doses administered at an interval of 21 days have an efficacy of 79 against symptomatic SARS-CoV-2 infection 14 or more days after the second dose. C and D Vero cells were infected with MVA-SARS-2-S MVA-S at a MOI of 10 and collected at indicated time points.
While AstraZeneca claimed that the vaccine was 70-percent effective it was later disclosed that the effectiveness was 62 percent in people who received two full doses and closer to. Vero CCL-81 cells were initially grown in tissue culture flasks and cell stacks using Dulbeccos Modified Eagle Medium DMEM Sigma-Aldrich India containing 10.

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet
Https Www Who Int Immunization Policy Position Papers Je Grad Inactivated Effectiveness Pdf

Efficacy And Safety Of An Inactivated Whole Virion Sars Cov 2 Vaccine Coronavac Interim Results Of A Double Blind Randomised Placebo Controlled Phase 3 Trial In Turkey The Lancet
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 4 Sage29apr2021 Sinovac Pdf

Interim Estimates Of Vaccine Effectiveness Of Bnt162b2 And Mrna 1273 Covid 19 Vaccines In Preventing Sars Cov 2 Infection Among Health Care Personnel First Responders And Other Essential And Frontline Workers Eight U S Locations December

Who Approves China S Sinopharm Covid 19 Vaccine For Emergency Use Has 79 Efficacy Coronavirus Outbreak News

Who Lists Sinopharm S Covid 19 Vaccine For Emergency Use
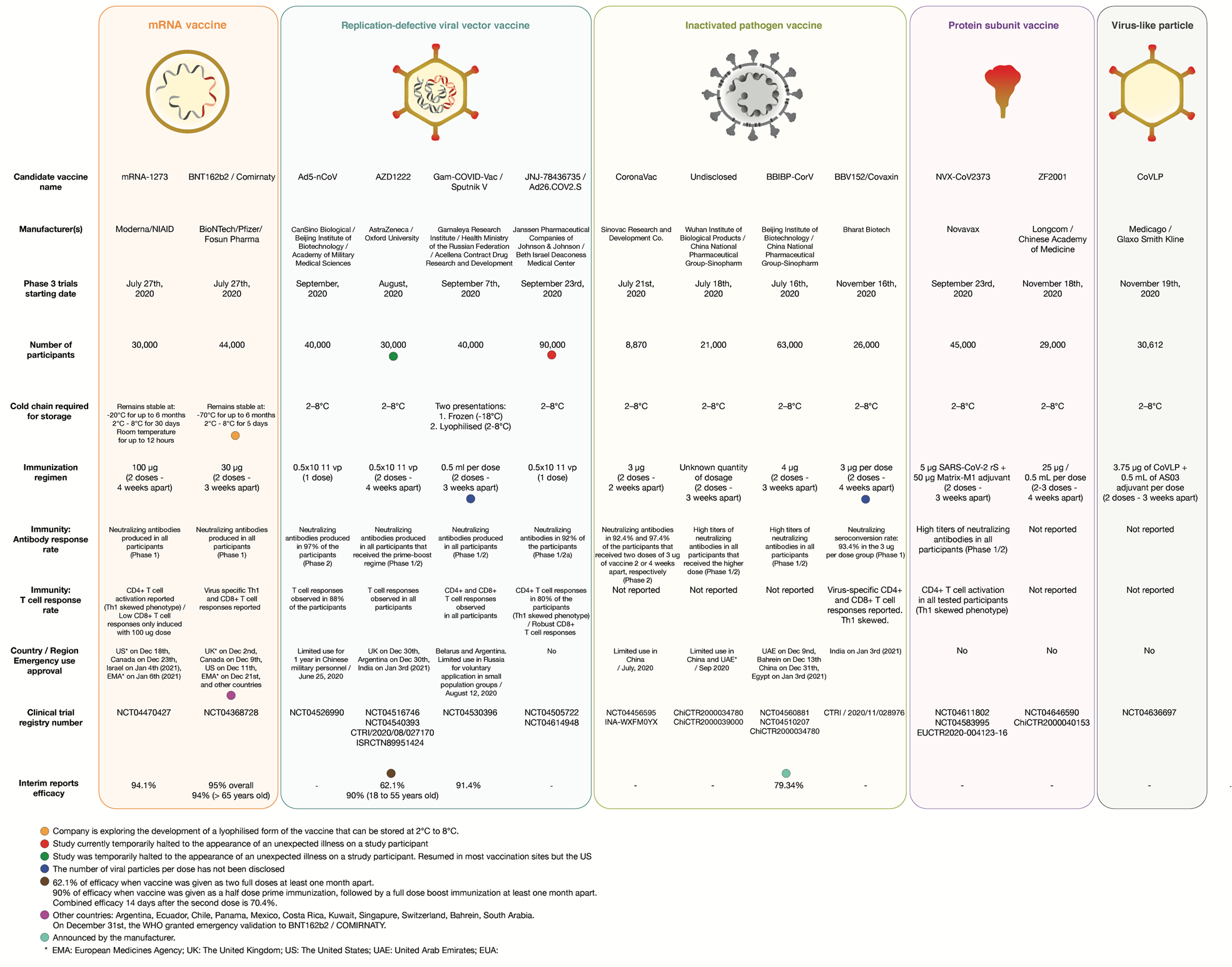
Sars Cov 2 Vaccines Strategies A Comprehensive Review Of Phase 3 Candidates Npj Vaccines
Https Cdn Who Int Media Docs Default Source Immunization Sage 2021 April 1 Sage29apr2021 Sinopharm Pdf Sfvrsn Ddf0d841 5

Sinopharm Vero Cell Inactivated Covid 19 Vaccine
World Health Organization Who What S The Difference Between Covid 19 Vaccine Efficacy And Effectiveness Vaccine Efficacy Refers To How The Vaccine Performs In Ideal Conditions Controlled Clinical Trials Vaccine

Seychelles Brings Back Curbs Despite Vaccination Success Bbc News
China S Covid Vaccine From Sinopharm Is 86 Effective Uae Says
Coronavirus Covaxin Efficacy Is 81 Works Against Variants The Hindu
Sinopharm S Two Covid 19 Shots Effective Study Says Reuters
Vaccines Free Full Text Development And Evaluation Of Vero Cell Derived Master Donor Viruses For Influenza Pandemic Preparedness Html

Efficacy Of Sinopharm S Covid 19 Vaccines Proved Again In New Trials Cgtn

Efficacy Safety And Immunogenicity Of A Vero Cell Culture Derived Trivalent Influenza Vaccine A Multicentre Double Blind Randomised Placebo Controlled Trial The Lancet

Efficacy Of The Chadox1 Ncov 19 Covid 19 Vaccine Against The B 1 351 Variant Nejm
